MedicalDevice Manufacturing License is required to legally produce medical devices in
India. The application involves meeting strict regulatory guidelines,
submitting technical documents, and undergoing inspections. Agile Regulatory
simplifies this complex process by handling the paperwork, ensuring compliance,
and coordinating with regulatory authorities. Our expertise helps you get
licensed quickly and efficiently, allowing you to focus on manufacturing
quality devices. Contact us for smooth Medical Device Manufacturing License
registration today.
Medical Device Manufacturing License

Written by
agileregulatory
446 days ago
Related articles:
How Plus-Size Medical Scrubs Are Redefining Comfort for Modern Doctors
Healthcare professionals spend most of their day on their feet — caring, moving, assisting, and often juggling multiple tasks. In such a dynamic environment, comfort isn’t a luxury; it’s essential...
India Medical Devices Market Size, Product Segmentation and Forecast Report by 2033
The India medical
devices market size reached USD 18.02 Billion in
2024. The market is expected to grow to USD 30.64 Billion by
2033, exhibiting a growth rate (CAGR) of..
India Medical Devices Market Size, Product Segmentation and Forecast Report by 2033
The India medicaldevices market size reached USD 18.02 Billion in
2024. The market is expected to grow to USD 30.64 Billion by
2033, exhibiting a growth rate (CAGR) of 6.08%..
Medical Radiation Detection Market Comprehensive Analysis Forecast by 2033
The Global Medical Radiation Detection Market Report presents an in-depth analysis, merging qualitative and quantitative insights to provide a holistic understanding of the market's dynamics. The report evaluates growth drivers,..
Top Healthcare Uniform Suppliers in India
In the ever-evolving landscape of healthcare, uniforms have transformed from mere professional attire into essential symbols of hygiene, identity, and trust. As hospitals, clinics, and laboratories raise their standards for..
Sterile Medical Packaging Market Growth Potential 2025–2033: Segmentation, Innovations, and Competitive Landscape
The Global Sterile Medical Packaging Market Report 2025 offers a thorough and data-driven analysis of the Sterile Medical Packaging Market industry, covering key aspects such as market size, growth drivers,..
Medical Affairs Outsourcing Market Analysis: Services, Industry Breakdown & Regional Growth Trends
In recent years, the global Medical Affairs Outsourcing Market has undergone a transformative journey, fueled by evolving consumer demands, cutting-edge innovations, and an increasing focus on sustainability. Our comprehensive Medical..
Medical Affairs Outsourcing Market Analysis: Services, Industry Breakdown & Regional Growth Trends
In recent years, the global Medical Affairs Outsourcing Market has undergone a transformative journey, fueled by evolving consumer demands, cutting-edge innovations, and an increasing focus on sustainability. Our comprehensive Medical..
Medical Implants Market Report: Demand, Trends, Outlook and Forecast by 2033
The Global Medical Implants Market Report 2025 offers a thorough and data-driven analysis of the Medical Implants Market industry, covering key aspects such as market size, growth drivers, limitations, and..
Medical Device Analytical Testing Outsourcing Market: Strategic Insights, Opportunities, Statistics by 2033
The Global Medical Device Analytical Testing Outsourcing Market Report presents an in-depth analysis, merging qualitative and quantitative insights to provide a holistic understanding of the market's dynamics. The report evaluates..
Understanding Device Fingerprinting: How It Tracks You and Your Privacy
Every time we go online, the using a phone, a pill, or a laptop, we leave small traces in the background. These traces can create a kind of “digital fingerprint”..
3 Strategies for Fixing QBDBMgrN is not Running on This Device
There are times when you may encounter the “QBDBMgrN is not running on this device” message on the screen. It is one of the issues that takes place while accessing..
Medical Device Analytical Testing Outsourcing Market: Strategic Insights, Opportunities, Statistics by 2033
The Global Medical Device Analytical Testing Outsourcing Market Report presents an in-depth analysis, merging qualitative and quantitative insights to provide a holistic understanding of the market's dynamics. The report evaluates..
Medical Device Analytical Testing Outsourcing Market: Strategic Insights, Opportunities, Statistics by 2033
The Global Medical Device Analytical Testing Outsourcing Market Report presents an in-depth analysis, merging qualitative and quantitative insights to provide a holistic understanding of the market's dynamics. The report evaluates..
Understanding Cisco ISE Licensing: Base, Plus, Apex, and Device Administration
In modern enterprise networks, maintaining secure and efficient access control is a top priority. Cisco Identity Services Engine (ISE) plays a pivotal role in providing centralized identity management, network access..
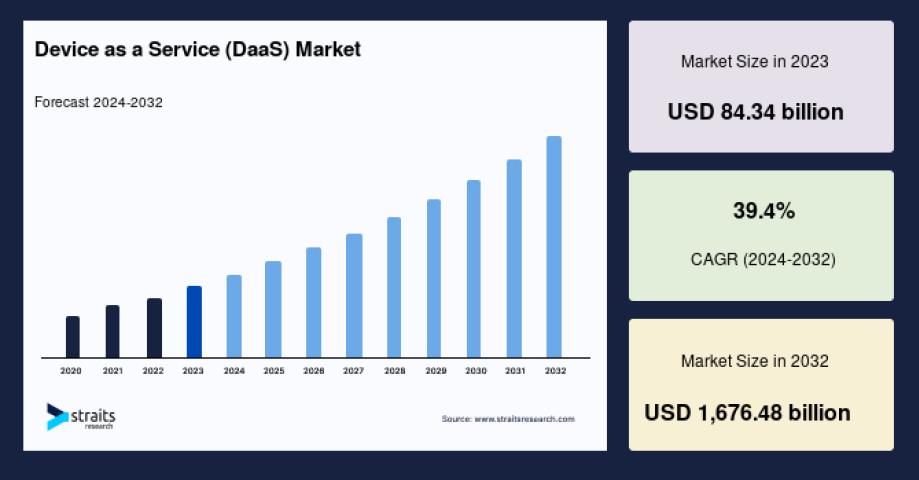
Global Device as a Service (DaaS) Market Trends: Rising Demand from IT & Telecom, BFSI, Healthcare, Manufacturing, and Government Sectors
The global Device as a Service (DaaS) Market is expanding rapidly as enterprises shift from traditional device ownership models to subscription-based service offerings, driven by the digital transformation wave and..
Access Medical Device Manufacturers Email List
DataCaptive’s Medical Device Manufacturers Email List gives you direct access to key decision-makers in the global medical device industry. Whether you’re promoting raw materials, software solutions, or healthcare equipment, this..
Access Medical Device Manufacturers Email List
DataCaptive’s Medical Device Manufacturers Email List gives you direct access to key decision-makers in the global medical device industry. Whether you’re promoting raw materials, software solutions, or healthcare equipment, this..